Welcome to part 2 of our 3-part discussion on cancer
commonalities! In this series we
are defining the features shared by most cancer cells as outlined in Hanahan
and Weinberg’s review article. It’s
a great introduction to what defines cancer. Let’s first recap our last discussion: In part 1 we learned about the first 3
hallmarks of cancer: sustained proliferative signaling, evading growth
suppressors, and resisting cell death.
Although distinct, these traits integrate several key signaling pathways
including the Ras-MAPK pathway, the PI3K-AKT axis, and the p53 tumor suppressor
pathway. Their intricate
interactions imply that alterations in one pathway can affect multiple pathways
and therefore, multiple cancer traits.
Figure 1:
Hallmarks of Cancer
In today’s discussion, we will introduce the 3 remaining
classical characteristics shared by cancer cells.
Enabling Replicative Immortality:
Normal cells are restricted in the number of times they can
divide and pass through the cell cycle by the length of telomeres at the end of
their chromosomes. Each time a
cell divides a telomere repeat is lost; and when telomeres become too short,
the cell undergoes apoptosis.
Those excluded from this limitation include stem or progenitor cells,
which inherently possess replicative immortality, and cancer cells which
acquire the ability to divide unconditionally. The main protein involved in maintaining the telomere is the
enzyme telomerase which acts by adding telomere repeats. Whereas this enzyme is practically
absent in normal, non-immortalized cells, in cancer cells this protein is
highly overexpressed and activated.
By adding telomere repeats to the end of chromosomes, it tricks the cell
into passing through unlimited cell cycle passages, enabling replicative
immortality.
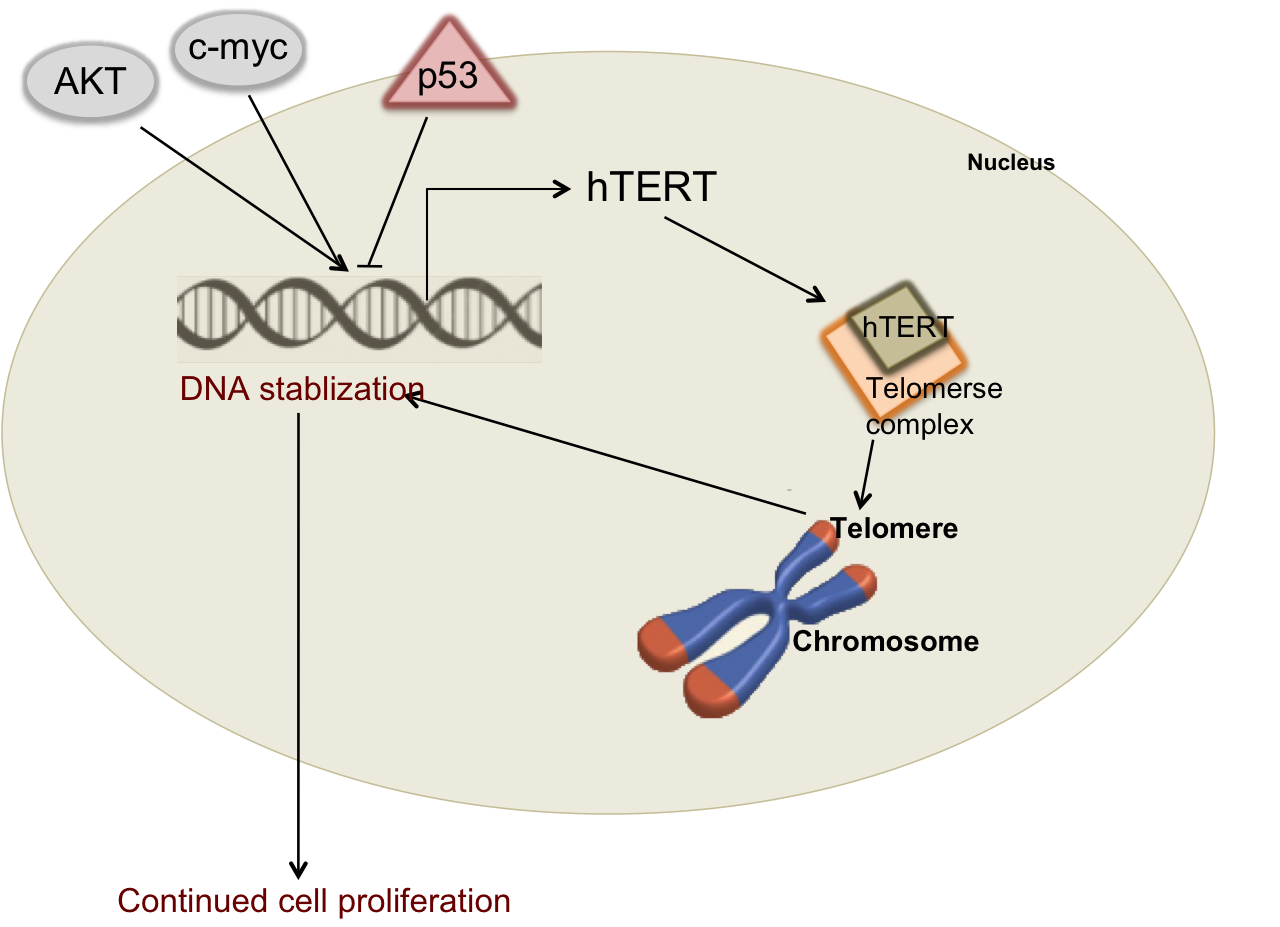
Figure 2: Regulation of Telomerase
Telomerase
is an enzyme complex with the key functional component called hTERT. hTERT can be transcriptionally
upregulated in cancer cells through hyper-activation of specific oncogenes
including AKT and c-myc or inhibited by the p53 tumor suppressor.
Inducing Angiogenesis:
Tumors, like any other organ in the body, require oxygen and
nutrients that blood carries for survival. The ability to sprout new blood vessels, termed
angiogenesis, which normally only occurs during embryonic and postnatal
development, also allows an aberrant cellular mass to develop into a detectable
tumor. And like most other
cellular processes, this process balances between the on/off states. The “angiogenic switch” is governed by
pro-angiogenic factors such as vascular endothelial growth factor (VEGF-A) and
inhibitors including thrombospondin-1 (TSP-1).
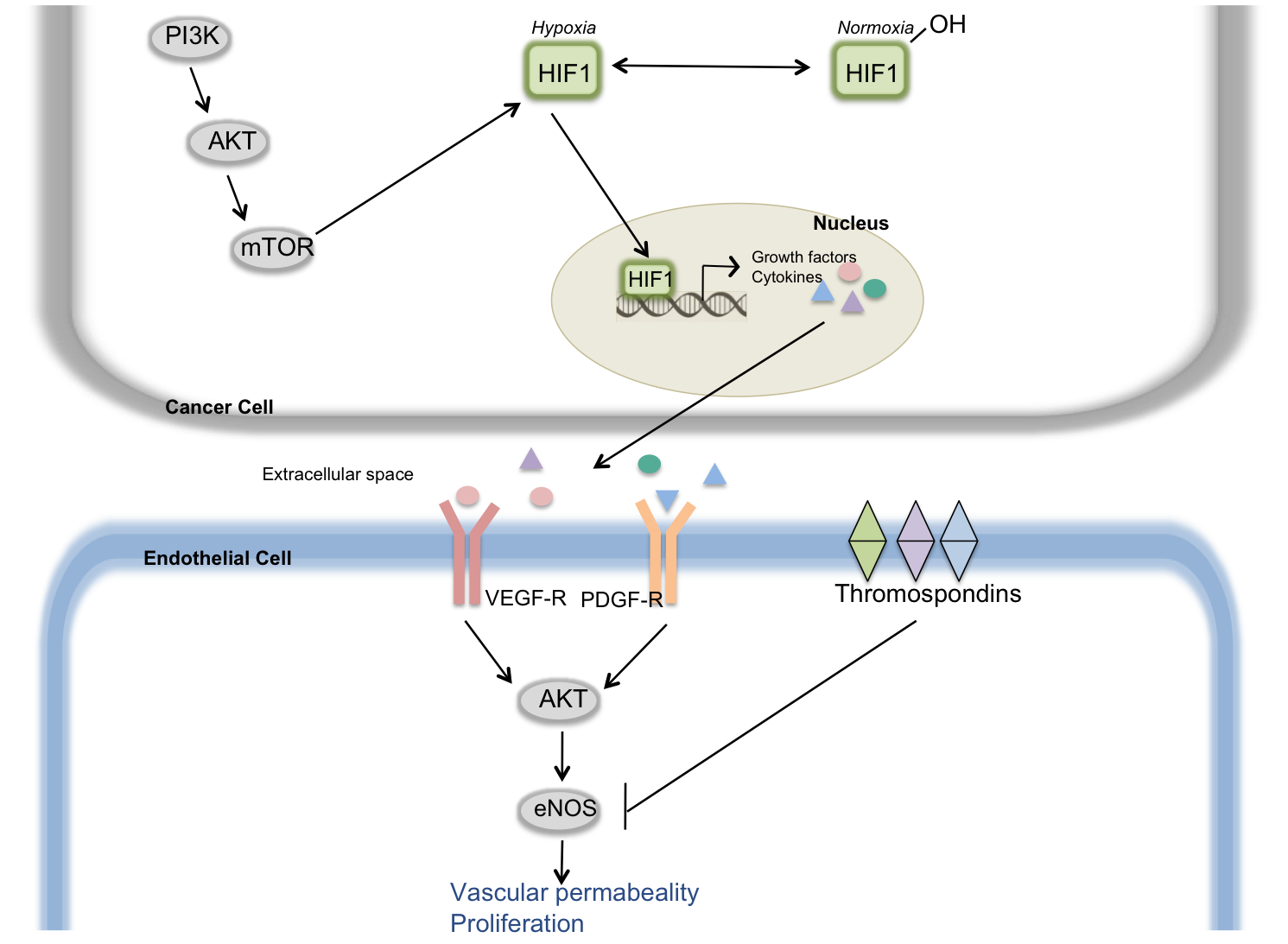
Figure 3: Angiogenic Switch
When
cells are well oxygenated, the HIF1 protein is hydoxyenated and inactive. Under hypoxic, or oxygen
starvation, conditions such as when a tumor is growing, HIF1 translocates to the
nucleus to induce transcription of growth factors and cytokines. One of these growth factors in VEGF
(Vascular Endothelial Growth Factor) which, after secretion into the
extracellular matrix can bind the VEGFR receptor on neighbouring endothelial
cells. This activates downstream
pathways including the AKT signaling pathway to enhance vascular permeability
and vascular growth, leading to the formation of new blood vessels. Endothelial cell signaling can be
inhibited by the thrombospondin family of cell surface receptors. The balance between vascular growth
factors and thrombospondins determines the balance of the angiogenic switch.
Activating Invasion and Metastasis:
Although the growth of a primary tumor represents a diseased
state, the development of metastases signals an advanced and aggressive disease
and is often associated with reduced mortality. The metastatic cascade involves a series of steps beginning
with local invasion into the surrounding environment, entry of cancer cells
into the blood or lymphatic systems (intravasation), transit and survival of
these migrating cells in the harsh fast-flowing streams of the blood or
lymphatic systems, exiting into surrounding tissue (extravasation), and finally
the formation of small cancerous nodules (micrometastases) and the growth of
these nodules into macroscopic lesions (colonization). The ability of cells to undergo this
process requires alterations in their morphology, most notably a change from an
epithelial (densely packed, with cell-cell contacts) to a mesenchymal
(migratory, elongated, and loss of cell-cell contact) phenotype. This transition occurs mainly through
activation of a transcriptional program controlled by a group of transcription
factors including Snail, Slug, and Twist.
These key players orchestrate most of the metastatic cascade.
Figure 4: Metastatic cascade (3)
Where do we go from
here?
Over the last two discussions, we have defined the classical
hallmarks of cancer. Although the
order in which cancer cells acquire these traits may differ, ultimately most
cancer cells will share these characteristics. For this reason, targeting a key hallmark may widely benefit
cancer patients as a whole group.
This is the case with conventional chemotherapy which acts by targeting
rapidly proliferating cells.
What also becomes apparent is how cancer (and probably most
disease) is a result of an imbalance.
Normal cells grow, divide, migrate, apoptose etc but under tight regulation in time and space – in other words,
with the proper balance. Cancer
cells tip this balance; they carry out these same normal cellular processes,
only without proper regulation.
Effective therapy aims to rebalance the cell to a homeostatic state.
References:
1 1. Hahahan D and Weinberg RA.
Hallmarks of Cancer Cell. 2001;100:57-70.
2 2. Hahahan D and Weinberg RA.
Hallmarks of Cancer: The Next Generation
Cell. 2011;144:646-74.
3. Fidler IJ. The pathogenesis of metastasis: The
‘seed and soil’ hypothesis revisited.
Nat. Rev. Cancer.
2003;3:453-8.

No comments:
Post a Comment